Health Food Regulations in China

China is one of the most promising and developed countries in the world. Thus, it represents a wonderful market to conquer for foreign companies. Chinese consumers are more and more concerned about their health and are paying attention to the food they eat. Therefore, the health food industry has been in constant growth these past few years.
However, as it has generated a greater demand for health products, the Chinese government had to regulate this market in order to avoid sanitary and health issues for its citizens, by implementing strict regulations for companies wanting to sell health products in China.
Cost-Effective Agency
KPI and Results focused. We are the most visible Marketing Agency for China. Not because of huge spending but because of our SMART Strategies. Let us help you with: E-Commerce, Search Engine Optimization, Advertising, Weibo, WeChat, WeChat Store & PR.
Why it’s Important to Comply With Health Food Regulations?
Consumer Protection
The regulations aim to protect consumers from unsafe, substandard, or misleading health food products. By setting strict standards and requirements for registration, manufacturing, and labeling, the regulations help ensure that imported health foods meet the food safety law of China.
Product Quality and Safety
Health food regulations establish guidelines and requirements for manufacturing practices, ingredient sourcing, quality control systems, and testing procedures. These measures help maintain the quality and safety of healthy food products throughout the supply chain.
Mitigating Health Risks
Health foods often contain bioactive substances and may interact with medications or have potential adverse effects if misused. Through the registration process, health food regulations enable regulatory authorities to assess the safety and potential risks associated with these products, ensuring that they are suitable for consumption.
Consumer Confidence
Clear and accurate labeling requirements, claims restrictions, and advertising guidelines promote transparency and prevent misleading or exaggerated claims about health food products. This helps build consumer trust and confidence in the industry and allows consumers to make informed decisions about the products they purchase.
Industry Development
Well-regulated health food regulations provide a stable and predictable business environment for manufacturers, importers, and distributors. By ensuring compliance, these regulations support the growth and development of the health food industry in China.
International Trade
Compliance with health food regulations is crucial for international trade. Aligning with global standards and requirements helps facilitate the import and export of health food products, promoting trade relationships with other countries.
The Health Food Market in China
In China, health food and products are usually defined as products that have specific health functions or supply vitamins or minerals. With the goal of regulating the body’s function, healthy food can be considered suitable for specific groups of people. However, the purpose is to cure disease and should not have any acute, sub-acute, or chronic health effects on one’s body.
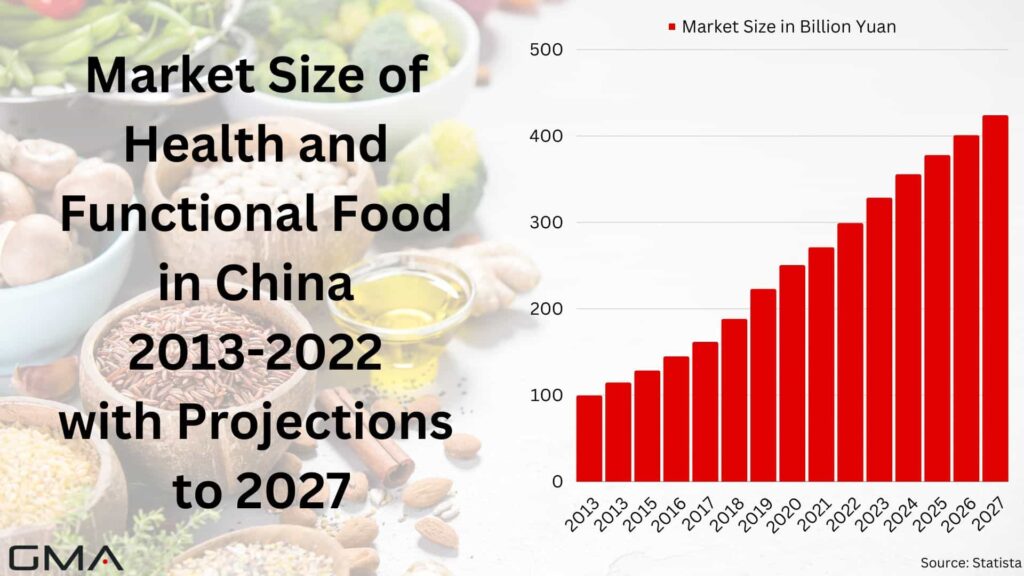
In 2022, China’s functional foods market experienced significant growth, reaching approximately 298.9 billion yuan, an increase from the previous year’s 270.8 billion yuan. This expansion can be attributed to several factors, including rising disposable incomes and increasing awareness of the importance of a balanced and healthy diet.
As more Chinese consumers prioritize their well-being, the demand for functional foods has surged in recent years. This trend reflects a growing desire to maintain a healthy lifestyle and underscores the significance of the functional foods industry in China. Looking ahead, the market is expected to continue its upward trajectory, with positive projections until 2027.
Who are Chinese health food consumers?
Following some serious scandals in the Chinese food market, Chinese people have become more concerned than before when it comes to food, especially in terms of health products. The Chinese government considers this market a serious and important issue, and that’s why many regulations were introduced these past few years.
The purpose of these regulations is to avoid any health safety issues and scandals. The main target of these health products is the elderly audience. According to a survey from the China Healthcare Association, the elderly account for more than 50% of the health food market.
The Health Food Market During the Covid-19 Pandemic
For many Chinese consumers, eating health products is a simple way to improve their general health and well-being, meeting nutritional deficits and providing a feeling of self-medication (even if it is not the purpose).
During Covid-19, for example, a lot of people were purchasing health products in order to ‘protect themselves from being infected and for their immune systems. Moreover, given the emphasis on traditional medicine in China, vitamins and herbs are highly appreciated by the majority of the Chinese population.
Regulatory Authorities
State Administration for Market Regulation (SAMR)
The State Administration for Market Regulation (SAMR) is a key regulatory authority in China responsible for health food regulations. SAMR formulates policies, standards, and guidelines related to health food registration, manufacturing, labeling, and advertising. It plays a central role in ensuring consumer safety and product quality in the health food industry.
China Food and Drug Administration (CFDA)
The China Food and Drug Administration (CFDA) is another important regulatory authority involved in health food regulations, particularly for products with functional claims. CFDA works closely with SAMR to establish standards and enforce regulations related to functional health foods. It contributes to the evaluation of efficacy, safety, and claims of healthy food products, aiming to protect consumer interests.
National Health Commission (NHC)
The National Health Commission (NHC) collaborates with SAMR and CFDA to set standards and guidelines for health food regulations. NHC focuses on public health and plays a significant role in ensuring that healthy food products meet the necessary health and food safety standards. It works in conjunction with other regulatory authorities to promote consumer well-being and protect public health interests.
Other Relevant Regulatory Bodies
In addition to SAMR, CFDA, and NHC, other regulatory bodies may play a role in health food regulations in China. These bodies could include local health departments, trade associations, and research institutions. They provide expertise, guidance, and support in specific areas related to health food regulation and supervision.
Overall, these regulatory authorities, including SAMR, CFDA, and NHC, along with other relevant bodies, work collectively to establish and enforce health food regulations in China. Their collaboration ensures the safety, efficacy, and quality of health food products and helps create a favorable regulatory environment for the health food industry.
Registration and Approval Process
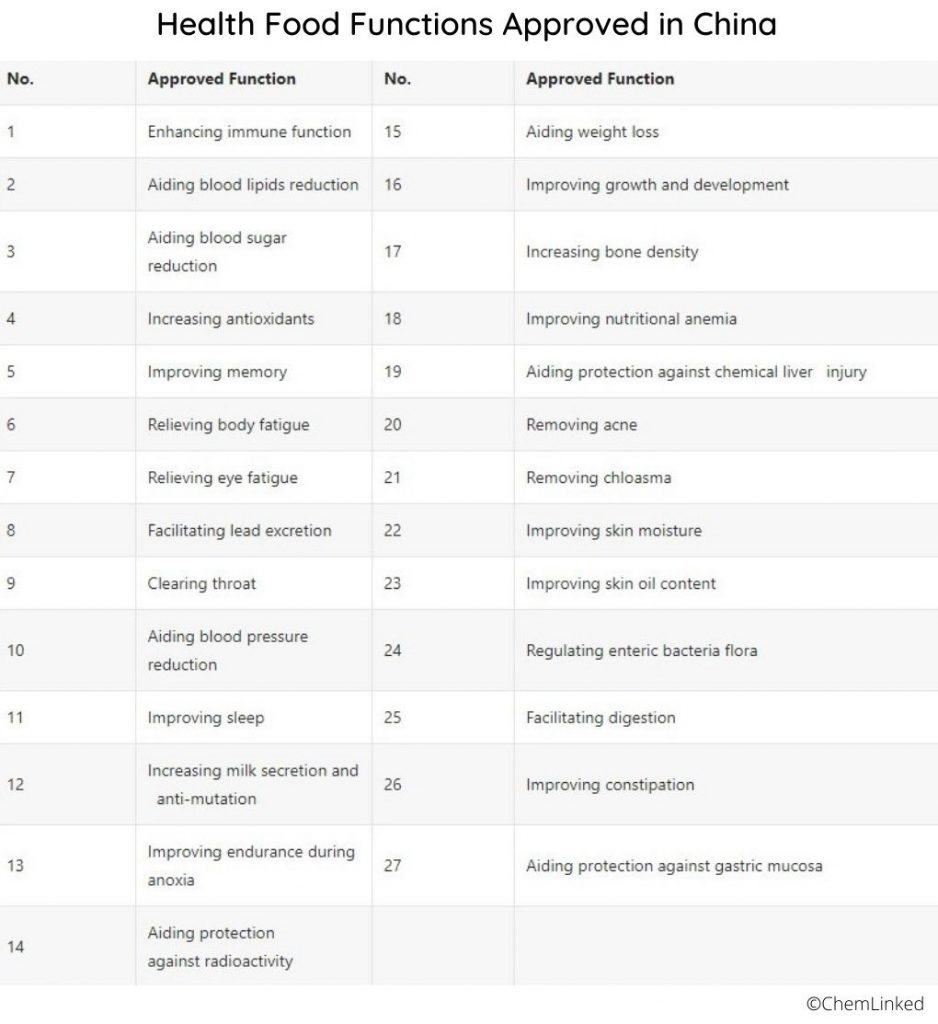
Categories of Health Foods
Health foods in China are classified into different categories:
- dietary supplements
- functional foods
- traditional Chinese medicines.
Each category may have specific requirements and regulations.
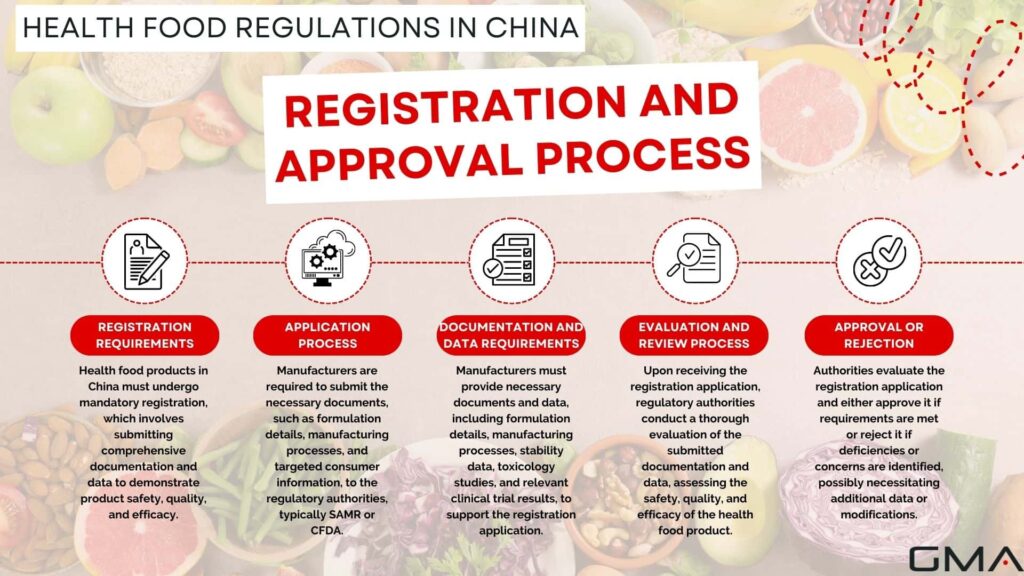
Registration Requirements
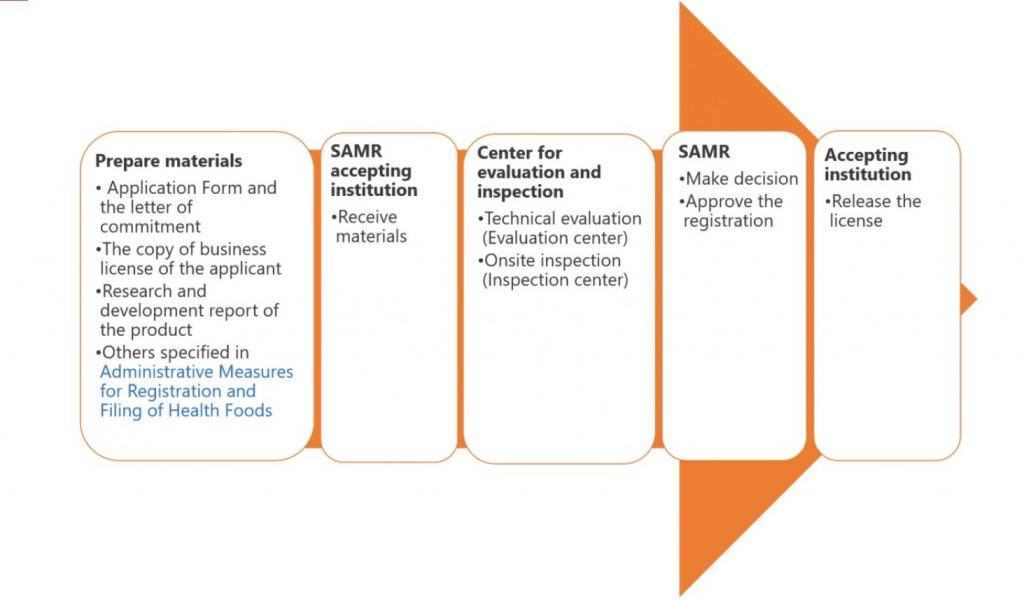
Registration is mandatory for health food products before they can be legally marketed in China. The registration requirements include submitting comprehensive documentation and data to support the safety, quality, and efficacy of the product. This may include information about the product formula, production process, raw materials, and relevant scientific research.
Application Process
The application process for health food registration involves submitting the necessary documents to the regulatory authorities, typically SAMR or CFDA. The application should provide clear and accurate information about the product, its intended use, and the targeted consumer group.
Documentation and Data Requirements
To support the registration application, manufacturers need to provide various documents and data. This may include product formulation details, manufacturing process descriptions, stability data, toxicology studies, and clinical trial results (if applicable). The documentation should be comprehensive, scientifically sound, and meet the regulatory requirements.
Evaluation and Review Process
Upon receiving the registration application, the regulatory authorities evaluate the submitted documentation and data. The evaluation process involves assessing the safety, quality, and efficacy of the health food product. The authorities may conduct thorough scientific reviews, consult expert panels, and request additional information if needed.
Approval or Rejection of Registration
After the evaluation and review process, the regulatory authorities make a decision regarding the registration application. If the product meets all the necessary requirements and demonstrates safety, quality, and efficacy, it may receive approval for registration. However, if any deficiencies or concerns are identified, the authorities may reject the registration application or request additional data or modifications to address the issues.

Labeling and Packaging Requirements
- Mandatory labeling information: China’s health food regulations mandate essential labeling information on product packaging, including the product name, ingredients, food additives, net content, shelf life, storage conditions, and manufacturer details. Clear and accurate labeling is crucial for providing consumers with important product information.
- Nutritional labeling: Health food regulations require nutritional labeling on packaging, providing information on energy, protein, fat, carbohydrates, and other relevant nutrients. This promotes transparency and helps consumers make informed choices about the nutritional content of the health food product.
- Claims and advertising restrictions: To prevent misleading claims, health food regulations in China impose restrictions on claims and advertising. Claims must be substantiated by scientific evidence and should not deceive consumers. Advertising should not make false or deceptive statements about the health benefits of the product. These restrictions ensure consumer protection and promote honest marketing practices.
- Language requirements: Health food labeling in China must be in the Chinese language to ensure consumer understanding. Clear and concise language is essential for facilitating comprehension and avoiding confusion among consumers.
- Packaging guidelines: Health food regulations provide packaging guidelines, specifying requirements for materials, size, labeling placement, and usage instructions. These guidelines aim to ensure proper presentation, protection, and information delivery to consumers.

Manufacturing and Quality Control Standards
Good Manufacturing Practices (GMP)
Health food regulations in China require manufacturers to adhere to Good Manufacturing Practices (GMP). GMP guidelines establish standards for facility design, equipment maintenance, hygiene practices, personnel training, and overall manufacturing processes. Compliance with GMP ensures that healthy food products are produced in a controlled and sanitary environment.
Quality Control Systems
Manufacturers are expected to implement robust quality control systems to ensure the consistent quality of healthy food products. This involves establishing procedures for raw material evaluation, in-process control, and finished product testing. Quality control systems help identify and address any deviations or inconsistencies during the manufacturing process.
Ingredient Sourcing and Traceability
Health food regulations emphasize the importance of ingredient sourcing and traceability. Manufacturers must source ingredients from approved suppliers and maintain records to demonstrate the origin and authenticity of the ingredients used. Traceability measures help ensure the quality and safety of healthy food products throughout the supply chain.

Testing and Analysis Requirements
Manufacturers are required to conduct testing and analysis of healthy food products to verify compliance with regulatory standards. This includes testing for ingredient authenticity, potency, microbiological contamination, and other relevant parameters. Regular product testing helps maintain the quality and safety of healthy food products.
Quality Management Systems
Health food manufacturers should establish comprehensive quality management systems to monitor and control all aspects of production. This involves implementing procedures for quality assurance, documentation control, deviation management, and product release. Quality management systems help ensure that healthy food products meet the required quality standards consistently.
Post-Market Surveillance and Monitoring
- Adverse event reporting: Manufacturers and distributors must report adverse events associated with health food products to regulatory authorities, enabling timely interventions to protect consumer health.
- Product recalls and withdrawals: Regulatory authorities can initiate recalls or withdrawals of non-compliant or potentially harmful health food products, ensuring consumer safety and industry integrity.
- Market surveillance and inspections: Regular inspections and surveillance activities are conducted to monitor compliance and identify non-compliant products or practices in the health food market.
- Compliance and enforcement measures: Penalties, fines, and legal actions are implemented against non-compliant manufacturers or distributors, promoting adherence to health food regulations and deterring non-compliance.
Import and Export Regulations
Import and export of health foods in China are subject to specific regulations to ensure compliance and safety. Importers must obtain approvals and permits from regulatory authorities like SAMR and CFDA, providing documentation such as product registration certificates and safety assessments.
Exporters must adhere to destination country regulations, including labeling and safety compliance, with proper documentation. Both the import and export of food involve customs clearance procedures, and compliance with customs requirements is necessary.
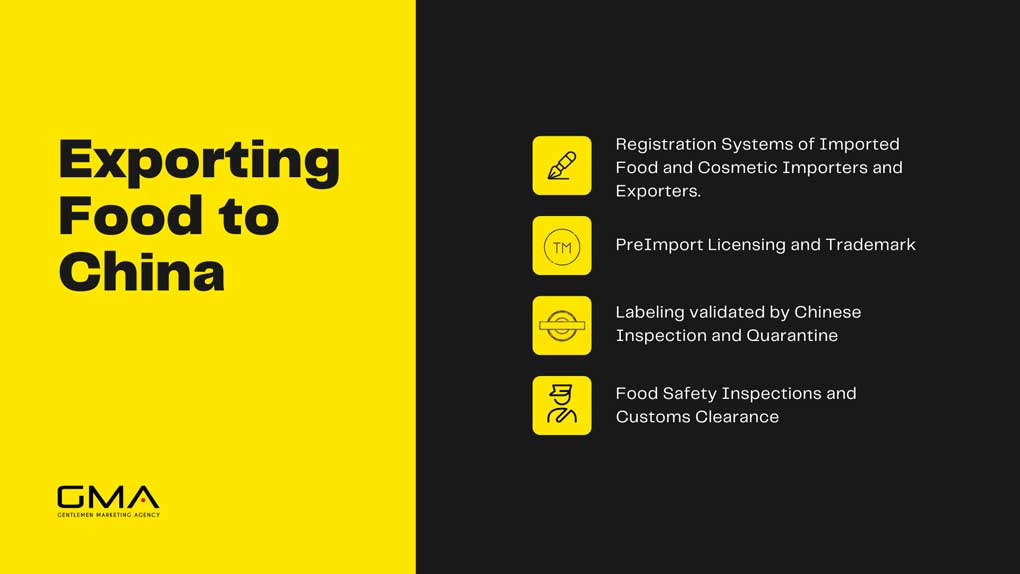
Adherence to international standards and certifications, such as ISO or organic certifications, can enhance product credibility and facilitate international trade. Compliance with these regulations and standards is essential for smooth import and export operations and to meet regulatory requirements domestically and internationally.
Recent Updates and Future Developments
Health food regulations in China are subject to periodic updates and amendments to keep pace with scientific advancements and industry developments. Recent updates may include changes in registration requirements, labeling guidelines, manufacturing standards, or claims and advertising restrictions. These amendments aim to enhance consumer safety, improve industry practices, and align with evolving scientific knowledge.
The health food industry in China is experiencing various emerging trends and challenges. These may include the emergence of new ingredients, innovative product formats, and advancements in technology.
Additionally, changing consumer preferences, increased demand for personalized nutrition, and rising awareness of sustainability pose challenges and opportunities for health food import. Adapting to these trends and addressing the associated challenges is crucial for industry growth and success.
China is actively involved in international harmonization efforts regarding health food regulations. This includes collaboration with international regulatory bodies and participation in discussions to align its regulations with global standards.
Harmonization aims to facilitate trade, ensure consistent product quality, and enhance international cooperation in the health food industry. By aligning with international standards, China can strengthen its position in the global market and promote cross-border trade and cooperation.
Explore Partnership Opportunities
Health food regulations in China encompass various aspects, including registration and approval processes, labeling and packaging requirements, manufacturing and quality control standards, post-market surveillance, and import and export regulations. These regulations aim to ensure consumer safety, product quality, and transparency in the health food industry. Compliance with these regulations is crucial for manufacturers, importers, and distributors operating in the Chinese market.
Compliance with health food regulations is of utmost importance to ensure consumer trust, maintain market access, and promote business sustainability. Manufacturers and other industry stakeholders must stay updated with the evolving regulatory landscape, including amendments and updates, to ensure continued compliance and to adapt to changing requirements. Staying informed helps businesses navigate the complex regulatory environment, mitigate risks, and maintain a competitive edge.

Adhering to health food regulations in China can have a positive impact on the health food industry as it fosters consumer confidence, promotes product quality, and supports industry growth. Businesses that comply with these regulations are well-positioned to meet consumer expectations and contribute to the overall development of the health food market.

Our agency, with over 20 years of experience in the field, is dedicated to assisting businesses in navigating the complexities of health food regulations in China. We offer expertise, guidance, and support in areas such as registration, compliance, labeling, and market access. Businesses interested in our services can contact us for tailored assistance and solutions to meet their specific needs.